
Hydroxychloroquine displayed at a pharmacy in Utah, May 27, 2020. Photo: Reuters/George Frey.
The New England Journal of Medicine published a study on June 3, 2020, that has implications for the position of the Indian Council of Medical Research vis-à-vis the use of hydroxychloroquine as a prophylactic against COVID-19. The visual summary (below), prepared by Dr Shriprakash Kalantri, an internal-medicine specialist at the Mahatma Gandhi Institute of Medical Sciences, Sevagram, sums up the study’s design and principal conclusions.
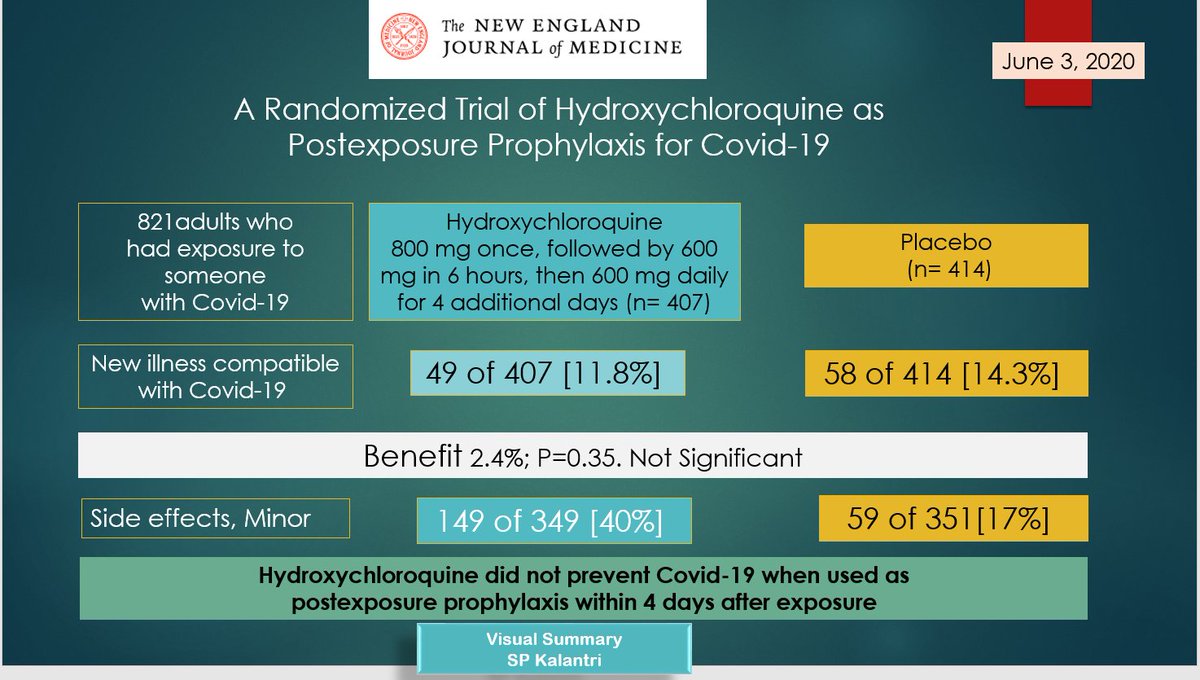
The following is a simplified annotation of the summary:
For the study, researchers selected 821 adults who had been exposed to someone with COVID-19. They were divided into two groups: 407 were put in the treatment group, which received hydroxychloroquine, and 414 joined the placebo group, whose members didn’t receive the drug but thought they did (i.e. to motivate the placebo effect). Of these people, 49 in the treatment group (11.8%) and 58 in the placebo group (14.3%) developed an illness “compatible with COVID-19”.
The study’s treatment regimen was as follows:
1. 800 mg of hydroxychloroquine on the first day, the ‘loading dose’
2. Six hours later, 600 mg of hydroxychloroquine
3. For the next four days, 600 mg of hydroxychloroquine daily
At the end of the study, the researchers reported that the number of patients who received hydroxychloroquine and developed COVID-19 was 2.4 percentage points less than the number of those who didn’t receive hydroxychloroquine and developed COVID-19. However, this result was statistically not significant enough to be considered notable.
Next, the researchers also reported that 40.1% of patients in the treatment group and 16.8% of patients in the placebo group developed minor side effects after taking the drug. They add in their paper that “no serious adverse reactions were reported”.
In conclusion, according to the researchers, “Hydroxychloroquine did not prevent COVID-19 when used as post-exposure prophylaxis within four days after exposure.”
ICMR has advised the use of hydroxychloroquine as a prophylactic to be taken before the potential onset of COVID-19, i.e. pre-exposure. The study tested for the drug’s effects as a post-exposure prophylactic against COVID, so we will have to wait for clinical trials that test the latter as well before we can comment specifically on the ICMR’s policy.
This said, as an editorial accompanying the study paper in the journal concluded, “The results reported … are more provocative than definitive, suggesting that the potential prevention benefits of hydroxychloroquine remain to be determined.”
The image was originally published by Dr Kalantri as a tweet. It has been republished here with his permission.

